Gastroparesis Market Poised for Extraordinary Growth at a 21.9% CAGR in the US by 2034 Due to the Launch of Novel MoAs Such as NK-1R Antagonists, 5-HT4 Receptor Agonists, D2 Receptor Antagonists, and Others | DelveInsight

The gastroparesis market is poised for significant growth, driven by the emergence of novel therapeutic classes such as the Dopamine D2 receptor antagonist (GIMOTI). Meanwhile, novel therapies like Neurokinin-1 Receptor (NK-1R) antagonist (tradipitant), 5-hydroxytryptamine-4 (5-HT4) receptor agonist (PCS12852), and others that address the limitations of existing treatments. The development of more effective and better-tolerated drugs is expected to enhance clinical utility.
New York, USA, Oct. 27, 2025 (GLOBE NEWSWIRE) — Gastroparesis Market Poised for Extraordinary Growth at a 21.9% CAGR in the US by 2034 Due to the Launch of Novel MoAs Such as NK-1R Antagonists, 5-HT4 Receptor Agonists, D2 Receptor Antagonists, and Others | DelveInsight
The gastroparesis market is poised for significant growth, driven by the emergence of novel therapeutic classes such as the Dopamine D2 receptor antagonist (GIMOTI). Meanwhile, novel therapies like Neurokinin-1 Receptor (NK-1R) antagonist (tradipitant), 5-hydroxytryptamine-4 (5-HT4) receptor agonist (PCS12852), and others that address the limitations of existing treatments. The development of more effective and better-tolerated drugs is expected to enhance clinical utility.
DelveInsight’s Gastroparesis Market Insights report includes a comprehensive understanding of current treatment practices, emerging gastroparesis drugs, market share of individual therapies, and current and forecasted gastroparesis market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
Gastroparesis Market Summary
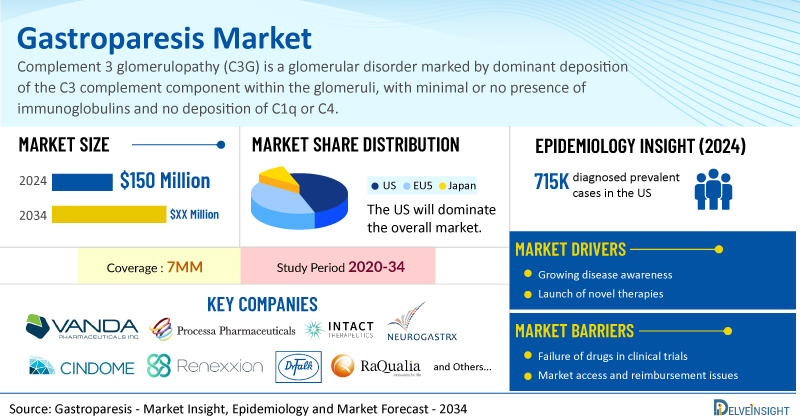
- The market size for gastroparesis was found to be USD 150 million in the US in 2024.
- The growing use of GLP-1 agonists, such as OZEMPIC and WEGOVY, is contributing to new gastroparesis cases by slowing gastric emptying and increasing patient risk.
- In 2024, the US recorded 715,000 diagnosed prevalent cases of gastroparesis, projected to grow by 2034.
- Key gastroparesis companies, including Vanda Pharmaceuticals, Processa Pharmaceuticals, Intact Therapeutics, Neurogastrx, CinDome Pharma, Renexxion Ireland, Dr. Falk Pharma GmbH, RaQualia Pharma, Aclipse Therapeutics, Chong Kun Dang Pharmaceuticals, and others, are actively working on innovative Gastroparesis drugs.
- Some of the key gastroparesis therapies in clinical trials include Tradipitant, PCS12852, NG101 (metopimazine), CIN-102 (deudomperidone), Naronapride (ATI-7505), RQ-00000010, Lobeglitazone, and others. These novel gastroparesis therapies are anticipated to enter the gastroparesis market in the forecast period and are expected to change the market.
- As per DelveInsight’s analysis, by 2034, among the therapies, the highest revenue is expected to be generated by antiemetic agents in the United States.
- Vanda Pharmaceuticals’ tradipitant is expected to entered the US market by 2027.
Discover which gastroparesis medications are expected to grab the market share @ Gastroparesis Market Report
Key Factors Driving the Growth of the Gastroparesis Market
Rising Gastroparesis Prevalence
According to DelveInsight analysis, the United States accounted for approximately 715K cases of gastroparesis in 2024. These cases are anticipated to increase by 2034. This upward trend is driven by the rising prevalence of diabetes, the leading underlying cause, improved diagnostic tools enabling more accurate detection, and greater clinical awareness leading to higher diagnosis rates
GIMOTI’s Role in Optimizing Healthcare Efficiency
GIMOTI is the only approved drug demonstrated to reduce hospitalizations, emergency room visits, medical office visits, and associated healthcare costs compared to oral metoclopramide, providing a clear clinical and economic advantage.
Clinical Promise of Naronapride
Naronapride uniquely integrates 5-HT4 receptor agonist with D2 receptor antagonism within a single molecule. Given its promising clinical profile and the growing unmet need in gastrointestinal disorders, there is a compelling opportunity for Renexxion Ireland and Dr. Falk Pharma GmbH to expand its research into dual-targeted therapeutic approaches.
Novel Gastroparesis Competitive Landscape
Companies like Vanda Pharmaceuticals (Tradipitant), Neurogastrx (NG101), CinDome Pharma (Deudomperidone), Renexxion Ireland (Naronapride), Processa Pharmaceuticals and Intact Therapeutics (PCS12852), and others are investigating their key products for gastroparesis.
Emergence of Novel Drug Classes
The pipeline of novel therapeutic candidates targeting diverse mechanisms such as NK-1R antagonists (Tradipitant), 5-HT4 receptor agonists (PCS12852), D2 receptor antagonists (NG101), dopamine D2/D3 receptor antagonists (CIN-102), and others demonstrates strong innovation and commitment to addressing unmet needs in gastroparesis treatment.
Gastroparesis Market Analysis
Managing gastroparesis remains challenging, focusing primarily on symptom relief and improving gastric motility rather than providing a definitive cure. Initial strategies often involve lifestyle modifications, including dietary adjustments, glycemic control, and adequate hydration, but these measures generally yield only modest benefits for most patients. Pharmacologic treatments form the cornerstone of therapy: prokinetic agents such as metoclopramide, domperidone, and erythromycin are used to promote gastric emptying, while antiemetics (ondansetron, granisetron, phenothiazines) and neuromodulators (tricyclic antidepressants, SNRIs, delta ligands) help manage nausea, vomiting, and related symptoms.
GIMOTI, a nasal form of metoclopramide, offers a non-oral option for those with persistent symptoms despite oral therapy. Nevertheless, many patients continue to experience inadequate symptom control. In severe, treatment-resistant cases, advanced interventions like gastric electrical stimulation or G-POEM may be considered, though both are invasive, expensive, and supported by limited evidence. Overall, existing treatments reveal significant unmet needs, emphasizing the necessity for safer, more effective, and accessible therapeutic options for gastroparesis.
The therapeutic landscape for gastroparesis is gradually evolving as new candidates progress through development. Companies including Vanda Pharmaceuticals (tradipitant), CinDome Pharma (deudomperidone), Processa Pharmaceuticals (PCS12852), Renexxion Ireland, Dr. Falk Pharma GmbH (Naronapride), Neurogastrx (NG101), and others are actively investigating treatments aimed at improving symptom management and overall disease control across the 7MM.
Learn more about the gastroparesis treatment options @ Gastroparesis Treatment Market
Gastroparesis Competitive Landscape
Some of the gastroparesis drugs in clinical trials include Tradipitant (Vanda Pharmaceuticals), PCS12852 (Processa Pharmaceuticals and Intact Therapeutics), NG101 (Neurogastrx), Deudomperidone (CinDome Pharma), Naronapride (Renexxion Ireland and Dr. Falk Pharma GmbH), Lobeglitazone (Aclipse Therapeutics and Chong Kun Dang Pharmaceuticals), and others.
Vanda Pharmaceuticals’ Tradipitant, a small-molecule NK-1 receptor antagonist, is under investigation for the treatment of gastroparesis. It is a New Chemical Entity (NCE) and an investigational drug that has not yet received approval for any indication. Eli Lilly licensed the product in 2012. In September 2024, the US FDA issued a Complete Response Letter (CRL) declining approval of Vanda’s New Drug Application (NDA) for tradipitant for managing gastroparesis symptoms. Following the CRL, Vanda has been seeking additional regulatory review, including pursuing legal action against the FDA.
Processa Pharmaceuticals and Intact Therapeutics’ PCS12852, a selective 5-HT4 receptor agonist, has completed a Phase IIa trial showing promising safety, tolerability, and efficacy in patients with diabetic gastroparesis. The drug is designed to normalize gastric emptying while avoiding the cardiovascular and central nervous system side effects associated with older therapies in this class.
The anticipated launch of these emerging gastroparesis therapies are poised to transform the gastroparesis market landscape in the coming years. As these cutting-edge gastroparesis therapies continue to mature and gain regulatory approval, they are expected to reshape the gastroparesis market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for gastroparesis, visit @ Gastroparesis Medication
Recent Developments in the Gastroparesis Market
- In August 2025, Evoke Pharma announced the official issuance of a new US patent related to its product GIMOTI. The US Patent No. 12,377,064, covers the use of intranasal metoclopramide in patients with moderate to severe symptoms of gastroparesis, and has now been issued by the United States Patent and Trademark Office (USPTO).
- In June 2025, CinDome Pharma announced that the first participant had been dosed in the ENVISION GI Phase II clinical trial of CIN-102 in adults with idiopathic gastroparesis. Based on a strong safety signal and notable reductions in nausea and vomiting symptoms observed in a blinded interim analysis of the ongoing Phase II Envision3D study in patients with diabetic gastroparesis, CinDome has raised an additional USD 40 million of new capital to initiate this Phase II trial to investigate deudomperidone as a treatment for a broader population of patients with this chronic disease.
- In June 2025, Processa Pharmaceuticals announced that it had entered into a binding term sheet with Intact Therapeutics, granting Intact an exclusive option to license PCS12852.
- In May 2025, Renexxion Ireland, in collaboration with its partner Dr. Falk Pharma GmbH, announced the successful completion of patient enrollment for the global Phase IIb MOVE-IT study evaluating the safety and efficacy of naronapride for the treatment of gastroparesis. The MOVE-IT study achieved its target enrolment of 320 patients.
- In May 2025, Renexxion Ireland anticipates top-line results of naronapride in the second half of 2025.
- In April 2025, Vanda once again sought summary judgment on its gastroparesis New Drug Application (NDA); however, in July 2025, the Center for Drug Evaluation and Research issued a proposed order denying a hearing on the matter. As of now, the Company’s lawsuit against the FDA remains pending.
What is Gastroparesis?
Gastroparesis is a disorder characterized by delayed stomach emptying lasting at least three months without any physical blockage. It presents with symptoms such as nausea, vomiting, bloating, early fullness, and abdominal discomfort. The delayed or impaired gastric emptying arises from disruptions in gastrointestinal motility, a complex process involving the parasympathetic and sympathetic nervous systems, gastric smooth muscle, pacemaker cells in the stomach and intestines, and the pyloric sphincter. The most frequent cause is idiopathic, followed by diabetes-related, post-surgical, and post-infectious origins. Its prevalence has grown in recent decades, likely due to rising rates of diabetes, obesity, and certain medications. Clinically, symptoms often overlap with functional dyspepsia, and the two conditions frequently coexist. Diagnostic tests are crucial to confirm the presence of gastroparesis.
Gastroparesis Epidemiology Segmentation
The gastroparesis epidemiology section provides insights into the historical and current gastroparesis patient pool and forecasted trends for the leading markets. More than 50% of US patients have diabetic gastroparesis, while idiopathic cases account for 10%, influencing treatment approaches and healthcare utilization.
The gastroparesis market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets segmented into:
- Total Diagnosed Prevalent Cases of Gastroparesis
- Etiology-specific Cases of Gastroparesis
- Age-specific Cases of Gastroparesis
- Gender-specific Cases of Gastroparesis
- Total Cases of Gastroparesis by the Severity of Delayed Gastric Emptying
- Treated Cases of Gastroparesis
Download the report to understand gastroparesis management @ Gastroparesis Treatment Options
| Gastroparesis Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Gastroparesis Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| US Gastroparesis Market CAGR | 21.9% |
| Gastroparesis Market Size in the US by 2034 | ~USD 900 Million |
| Key Gastroparesis Companies | Vanda Pharmaceuticals, Processa Pharmaceuticals, Intact Therapeutics, Neurogastrx, CinDome Pharma, Renexxion Ireland, Dr. Falk Pharma GmbH, RaQualia Pharma, Aclipse Therapeutics, Chong Kun Dang Pharmaceuticals, Evoke Pharma, EVERSANA, ANI Pharmaceuticals, and others |
| Key Gastroparesis Therapies | Tradipitant, PCS12852, NG101 (metopimazine), CIN-102 (deudomperidone), Naronapride (ATI-7505), RQ-00000010, Lobeglitazone, GIMOTI, REGLAN, and others |
Scope of the Gastroparesis Market Report
- Gastroparesis Therapeutic Assessment: Gastroparesis current marketed and emerging therapies
- Gastroparesis Market Dynamics: Conjoint Analysis of Emerging Gastroparesis Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Gastroparesis Market Unmet Needs, KOL’s views, Analyst’s views, Gastroparesis Market Access and Reimbursement
Discover more about gastroparesis drugs in development @ Gastroparesis Clinical Trials
Table of Contents
| 1 | Gastroparesis Market Key Insights |
| 2 | Gastroparesis Market Report Introduction |
| 3 | Executive Summary of Gastroparesis |
| 4 | Key Events |
| 5 | Epidemiology and Market Forecast Methodology |
| 6 | Gastroparesis Market Overview at a Glance |
| 6.1 | Clinical Landscape (Analysis by Molecule Type, Phase, and Route of Administration [RoA]) |
| 6.2 | Market Share of Gastroparesis by Therapies (%) in the 7MM in 2024 |
| 6.3 | Market Share of Gastroparesis by Therapies (%) in the 7MM in 2034 |
| 7 | Disease Background and Overview |
| 7.1 | Introduction |
| 7.2 | Stages of Gastroparesis |
| 7.3 | Gastroparesis Symptoms |
| 7.4 | Gastroparesis Etiology |
| 7.5 | Gastroparesis Pathophysiology |
| 7.6 | Gastroparesis Differential Diagnosis |
| 7.7 | Gastroparesis Diagnosis |
| 7.8 | Gastroparesis Diagnostic Guidelines |
| 8 | Gastroparesis Treatment |
| 9 | Epidemiology and Patient Population |
| 9.1 | Key Findings |
| 9.2 | Assumptions and Rationale |
| 9.3 | Total Diagnosed Prevalent Cases of Gastroparesis in the 7MM |
| 9.4 | The United States |
| 9.4.1 | Total Diagnosed Prevalent Cases of Gastroparesis in the United States |
| 9.4.2 | Etiology-specific Cases of Gastroparesis in the United States |
| 9.4.3 | Age-specific Cases of Gastroparesis in the United States |
| 9.4.4 | Gender-specific Cases of Gastroparesis in the United States |
| 9.4.5 | Total Cases of Gastroparesis by the Severity of Delayed Gastric Emptying in the United States |
| 9.4.6 | Treated Cases of Gastroparesis in the United States |
| 9.5 | EU4 and the UK |
| 9.6 | Japan |
| 10 | Gastroparesis Patient Journey |
| 11 | Marketed Gastroparesis Therapy |
| 11.1 | Key Cross of Marketed Therapies |
| 11.2 | GIMOTI (metoclopramide): Evoke Pharma and EVERSANA |
| 11.2.1 | Product Description |
| 11.2.2 | Regulatory Milestones |
| 11.2.3 | Other Development Activities |
| 11.2.4 | Summary of Pivotal Trial |
| 11.2.5 | Safety and Efficacy |
| 11.2.6 | Analysts’ Views |
| 12 | Emerging Gastroparesis Drugs |
| 12.1 | Key Cross Competition |
| 12.2 | Tradipitant (VLY-686): Vanda Pharmaceuticals |
| 12.2.1 | Product Description |
| 12.2.2 | Other Development Activities |
| 12.2.3 | Clinical Development |
| 12.2.3.1 | Clinical Trial Information |
| 12.2.4 | Safety and Efficacy |
| 12.2.5 | Analyst Views |
| 12.3 | PCS12852 (YH12852): Processa Pharmaceuticals and Intact Therapeutics |
| 12.4 | NG101 (metopimazine): Neurogastrx |
| 12.5 | Deudomperidone (CIN-102): CinDome Pharma |
| 12.6 | Naronapride (ATI-7505): Renexxion Ireland and Dr. Falk Pharma GmbH |
| 12.7 | Lobeglitazone (M107): Aclipse Therapeutics and Chong Kun Dang Pharmaceuticals |
| 12.8 | RQ-00000010: RaQualia Pharma |
| 13 | Gastroparesis Market: 7MM Market Analysis |
| 13.1 | Key Findings |
| 13.2 | Gastroparesis Market Outlook |
| 13.3 | Conjoint Analysis |
| 13.4 | Key Gastroparesis Market Forecast Assumptions |
| 13.5 | Total Market Size of Gastroparesis in the 7MM |
| 13.6 | Total Market Size of Gastroparesis by Therapies in the 7MM |
| 13.7 | The United States Gastroparesis Market Size |
| 13.7.1 | Total Market Size of Gastroparesis in the United States |
| 13.7.2 | Total Market Size of Gastroparesis by Therapies in the United States |
| 13.8 | EU4 and the UK Gastroparesis Market Size |
| 13.9 | Japan Gastroparesis Market Size |
| 14 | Gastroparesis Market Unmet Needs |
| 15 | Gastroparesis Market SWOT Analysis |
| 16 | KOL Views on Gastroparesis |
| 17 | Gastroparesis Market Access and Reimbursement |
| 17.1 | United States |
| 17.2 | EU4 and the UK |
| 17.3 | Japan |
| 17.4 | Summary and Comparison of Market Access and Pricing Policy Developments in 2025 |
| 17.5 | Market Access and Reimbursement of Gastroparesis |
| 18 | Bibliography |
| 19 | Gastroparesis Market Report Methodology |
Related Reports
Gastroparesis Clinical Trial Analysis
Gastroparesis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key gastroparesis companies, including Neurogastrx, Inc., Dr. Falk Pharma GmbH, CinDome Pharma, Inc., Entero Therapeutics, Inc., RaQualia Pharma, among others.
Diabetic Gastroparesis Market
Diabetic Gastroparesis Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key diabetic gastroparesis companies, including Neurogastrx, Vanda Pharmaceuticals, Takeda, Processa Pharmaceuticals, Theravance Biopharma, Allergan Evoke Pharma Inc., Theravance Biopharma, Censa Pharmaceuticals, CinDome Pharma, Bird Rock Bio, among others.
Diabetic Gastroparesis Clinical Trial Analysis
Diabetic Gastroparesis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key diabetic gastroparesis companies, including Neurogastrx, Vanda Pharmaceuticals, Takeda, Processa Pharmaceuticals, Theravance Biopharma, among others.
Gastro-Esophageal Junction Neuroendocrine Tumor Market
Gastro-Esophageal Junction Neuroendocrine Tumor Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key GEJ-NET companies, including Novartis, Ipsen, Bristol-Myers Squibb, Merck, Pfizer, Amgen, Teva Pharmaceutical, Eli Lilly and Company, HUTCHMED, GSK, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Pipeline Assessment
Healthcare Licensing Services
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
CONTACT: Contact Us Shruti Thakur [email protected] +14699457679 www.delveinsight.com
 Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. NewIndiaObserver.com takes no editorial responsibility for the same.
Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. NewIndiaObserver.com takes no editorial responsibility for the same.
