Global Patient Monitoring Devices Market to Cross USD 110 Billion Mark by 2032 | DelveInsight

The patient monitoring devices market is witnessing significant expansion, fueled by the increasing incidence of chronic conditions like cardiovascular diseases, diabetes, and respiratory disorders. Furthermore, rapid technological progress, such as AI, IoT, and wearable solutions, combined with ongoing product innovations by leading market players, is driving growth by improving device performance, accessibility, and patient care outcomes.
New York, USA, Sept. 17, 2025 (GLOBE NEWSWIRE) — Global Patient Monitoring Devices Market to Cross USD 110 Billion Mark by 2032 | DelveInsight
The patient monitoring devices market is witnessing significant expansion, fueled by the increasing incidence of chronic conditions like cardiovascular diseases, diabetes, and respiratory disorders. Furthermore, rapid technological progress, such as AI, IoT, and wearable solutions, combined with ongoing product innovations by leading market players, is driving growth by improving device performance, accessibility, and patient care outcomes.
DelveInsight’s Patient Monitoring Devices Market Insights report provides the current and forecast market analysis, individual leading patient monitoring devices companies’ market shares, challenges, patient monitoring devices market drivers, barriers, trends, and key market patient monitoring devices companies in the market.
Patient Monitoring Devices Market Summary
- The global patient monitoring devices market size is projected to increase from USD 51.5 million in 2024 to USD 110.3 million by 2032, reflecting strong and sustained growth.
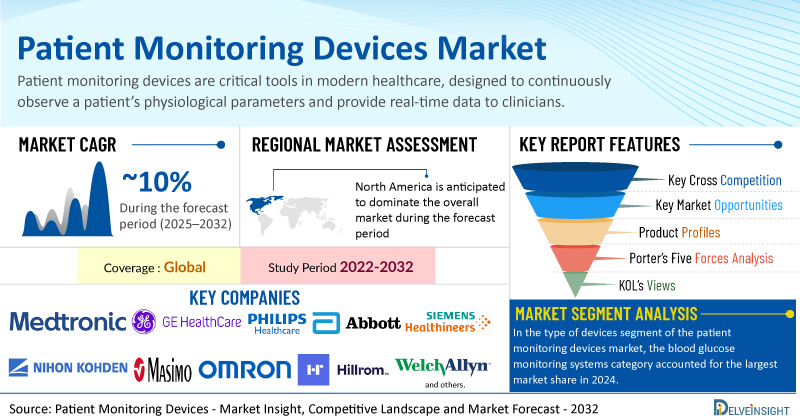
- The global patient monitoring devices market is expected to grow at a CAGR of ~10% during the forecast period from 2025 to 2032.
- The leading companies working in the patient monitoring devices market include Medtronic, GE Healthcare, Philips Healthcare, Abbott Laboratories, Siemens Healthineers, Nihon Kohden Corporation, Masimo Corporation, Omron Healthcare Co., Ltd., Smiths Medical, Hillrom, Welch Allyn, Inc., Natus Medical Incorporated, Mindray Medical International Limited, Nonin Medical, Inc., Spacelabs Healthcare, and others.
- Among all the regions, North America is anticipated to register the fastest growth in the patient monitoring devices market during the forecast period.
- In the type of devices segment of the patient monitoring devices market, the blood glucose monitoring systems category accounted for the largest market share in 2024.
To read more about the latest highlights related to the patient monitoring devices market, get a snapshot of the key highlights entailed in the Global Patient Monitoring Devices Market Forecast Report
Key Factors Contributing to the Rise in Growth of the Patient Monitoring Devices Market
Increasing Prevalence of Chronic Diseases
The rising incidence of chronic diseases such as cardiovascular disorders, diabetes, and respiratory conditions has significantly driven demand for patient monitoring devices. Continuous monitoring of vital signs and health parameters is crucial for timely intervention and improved patient outcomes, especially in aging populations with higher disease burdens.
Advancements in Healthcare Technology
Technological innovations in wearable devices, remote patient monitoring (RPM) systems, and wireless health solutions have enhanced the accuracy, reliability, and usability of patient monitoring devices. Integration of AI, IoT, and cloud-based platforms allows real-time data analysis and predictive healthcare, which fuels market growth.
Adoption of Telehealth and Remote Monitoring
The global shift toward telehealth, accelerated by the COVID-19 pandemic, has created a surge in demand for remote monitoring solutions. Patients can now track their health from home, reducing hospital visits and enabling healthcare providers to monitor patients continuously, which expands the market potential for monitoring devices.
Favorable Reimbursement Policies and Government Initiatives
Supportive reimbursement policies and healthcare funding by governments, particularly in developed regions, encourage the adoption of patient monitoring devices. Incentives for remote monitoring and preventive healthcare promote wider integration of these devices into healthcare systems.
Growing Home Healthcare and Self-Monitoring Trends
Rising awareness of self-care and home-based health management has increased consumer interest in devices like glucose monitors, pulse oximeters, and wearable ECG monitors. Convenience, affordability, and ease of use make home monitoring a viable alternative to frequent clinical visits.
Rising Healthcare Expenditure and Infrastructure Development
Increased spending on healthcare infrastructure, particularly in emerging markets, enables better access to advanced monitoring solutions. Investment in smart hospitals and clinics with integrated patient monitoring systems contributes to market expansion.
Get a sneak peek at the patient monitoring devices market dynamics @ Remote Patient Monitoring Devices Market Trends
Regional Patient Monitoring Devices Market Insights
In 2024, North America led the global patient monitoring devices market, capturing around 43% of the total share. This dominance is driven by a high prevalence of chronic diseases, widespread adoption of advanced healthcare technologies, and extensive use of remote patient monitoring supported by a mature telehealth infrastructure. The region’s leadership is further reinforced by favorable reimbursement policies, increasing healthcare spending, and the presence of major market players who drive innovation and adoption of monitoring solutions.
Technological advancements, such as wearable sensors, wireless connectivity, cloud platforms, and AI-powered analytics, are enabling real-time monitoring, predictive insights, and personalized patient care. Devices like continuous glucose monitors, smart ECG patches, and multi-parameter wearables empower patients to manage chronic conditions like diabetes and cardiovascular diseases from home, reducing hospital visits. For example, in July 2025, the FDA cleared the CardioTag, a wearable sensor that captures ECG, PPG, and SCG signals for comprehensive cardiac monitoring. Integration with smartphones and health apps, along with miniaturized, non-invasive designs and EHR compatibility, further improves usability, patient engagement, and clinical decision-making. Collectively, these factors are expected to drive significant growth in North America’s patient monitoring devices market.
In Europe, growth is fueled by advanced healthcare infrastructure, high adoption of digital health technologies, and supportive regulatory frameworks. Initiatives promoting telemedicine, remote monitoring, and home-based care are increasing the use of wearable and AI-enabled devices for managing chronic diseases such as diabetes and cardiovascular disorders. Regional manufacturers continue to innovate, offering solutions that enhance preventive and personalized care. For instance, in July 2025, Boston Scientific received CE Mark approval for its LATITUDE® Patient Management System, allowing remote monitoring of patients with implantable cardiac devices and facilitating timely clinical interventions.
Meanwhile, the Asia-Pacific is emerging as a key growth market due to its large patient population, rising chronic disease prevalence, and increasing healthcare expenditure. Rapid adoption of digital health solutions, government support for telemedicine and remote monitoring, and growing preventive care awareness are driving demand for wearable devices, continuous glucose monitors, and multi-parameter systems. Expanding healthcare infrastructure and rising investments from both global and local manufacturers are further accelerating market growth in the region.
To know more about why North America is leading the market growth in the patient monitoring devices market, get a snapshot of the Digital Patient Monitoring Devices Market Outlook
Recent Developmental Activities in the Patient Monitoring Devices Market
- In July 2025, Boston Scientific received CE Mark approval for its LATITUDE® Patient Management System, enabling remote monitoring of patients with implantable cardiac devices and facilitating timely clinical interventions.
- In July 2025, Cardiosense received FDA 510(k) clearance for its CardioTag device, a wearable sensor capable of simultaneously capturing electrocardiogram (ECG), photoplethysmogram (PPG), and seismocardiogram (SCG) signals, along with heart and pulse rate measurements.
- In June 2025, Cardinal Health launched the Kendall DL™ Multi System. This single-patient use monitoring cable continuously tracks cardiac activity, blood oxygen levels, and temperature, streamlining patient monitoring from admission to discharge.
- In April 2025, Dexcom secured FDA clearance for its G7 15-Day continuous glucose monitoring (CGM) system. This device offers an extended wear time of up to 15.5 days, enhancing convenience for diabetes patients.
- In February 2025, the FDA approved the Viatom O2Ring by Apnimed. This ring-shaped pulse oximeter continuously tracks blood oxygen saturation (SpO₂) and heart rate, with built-in vibration alerts for threshold breaches.
Patient Monitoring Devices Overview
Patient monitoring devices are critical tools in modern healthcare, designed to continuously observe a patient’s physiological parameters and provide real-time data to clinicians. These devices range from simple wearable sensors that track heart rate, blood pressure, and oxygen saturation, to sophisticated systems like multi-parameter monitors used in intensive care units (ICUs). By offering continuous monitoring, these devices enable early detection of clinical deterioration, helping healthcare providers intervene promptly and improve patient outcomes. Many patient monitoring devices now incorporate wireless technology, allowing remote monitoring and integration with electronic health records (EHRs) for seamless data tracking.
| Patient Monitoring Devices Market Report Metrics | Details |
| Coverage | Global |
| Study Period | 2022–2032 |
| Patient Monitoring Devices Market CAGR | ~10% |
| Patient Monitoring Devices Market Size by 2032 | USD 110.3 Million |
| Key Patient Monitoring Devices Companies | Medtronic, GE Healthcare, Philips Healthcare, Abbott Laboratories, Siemens Healthineers, Nihon Kohden Corporation, Masimo Corporation, Omron Healthcare Co., Ltd., Smiths Medical, Hillrom, Welch Allyn, Inc., Natus Medical Incorporated, Mindray Medical International Limited, Nonin Medical, Inc., Spacelabs Healthcare, and others |
Patient Monitoring Devices Market Assessment
- Patient Monitoring Devices Market Segmentation
- Patient Monitoring Devices Market Segmentation By Types of Devices: Neuromonitoring Devices, Cardiac Monitoring Devices, Respiratory Monitoring Devices, Hemodynamic Monitoring Devices, Blood Glucose Monitoring Systems, Multi-parameter Monitoring Devices, and Others
- Patient Monitoring Devices Market Segmentation By Application: Cardiology, Neurology, Respiratory, and Others
- Patient Monitoring Devices Market Segmentation By End-User: Hospitals & Clinics, Ambulatory Surgical Centers, Home Care Settings, and Others
- Patient Monitoring Devices Market Segmentation By Geography: North America, Europe, Asia-Pacific, and Rest of World
- Porter’s Five Forces Analysis, Product Profiles, Case Studies, KOL’s Views, Analyst’s View
Which MedTech key players in the patient monitoring devices market are set to emerge as the trendsetter explore @ Patient Monitoring Devices Market Analysis
Table of Contents
| 1 | Patient Monitoring Devices Market Report Introduction |
| 2 | Patient Monitoring Devices Market Executive Summary |
| 3 | Competitive Landscape |
| 4 | Regulatory Analysis |
| 5 | Patient Monitoring Devices Market Key Factors Analysis |
| 6 | Patient Monitoring Devices Market Porter’s Five Forces Analysis |
| 7 | Patient Monitoring Devices Market Layout |
| 8 | Patient Monitoring Devices Market Company and Product Profiles |
| 9 | KOL Views |
| 10 | Project Approach |
| 11 | About DelveInsight |
| 12 | Disclaimer & Contact Us |
Interested in knowing FDA-approved remote patient monitoring devices? Click to get a snapshot of the Patient Monitoring Devices Market Assessment
Related Reports
AI in Remote Patient Monitoring Market
AI in Remote Patient Monitoring Market Insights, Competitive Landscape, and Market Forecast – 2032 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key AI in remote patient monitoring companies, including Medtronic, iRhythm Inc., Koninklijke Philips N.V., Siemens Healthineers, GE HealthCare, Apple Inc., AliveCor Inc., Biofourmis, Optum, Inc., Headspace Health, Withings, NeuroRPM Inc., Caretaker Medical, Implicity, Stryker, Biobeat, among others.
Blood Glucose Monitoring Systems Market
Blood Glucose Monitoring Systems Market Insights, Competitive Landscape, and Market Forecast – 2032 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key blood glucose monitoring systems companies, including Medtronic, Abbott, F. Hoffmann-La Roche Ltd., Johnson & Johnson Services, Inc., Dexcom Inc., ARKRAY, Inc., Senseonics, WaveForm Technologies, Inc., B. Braun Melsungen AG, Ascensia Diabetes Care Holdings AG, Ypsomed, i-SENS, Inc., Intuity Medical, Inc., ACON Laboratories, Inc., Prodigy Diabetes Care, LLC, Sinocare, Rossmax International Ltd., Menarini Diagnostics s.r.l., Bionime Corporation, Nemaura, among others.
Cardiac Monitoring System Market
Cardiac Monitoring System Market Insights, Competitive Landscape, and Market Forecast – 2032 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key cardiac monitoring system companies, including Boston Scientific Corporation, Neurosoft, ACS Diagnostics, Medtronic, BIOTRONIK, Microport, GENERAL ELECTRIC COMPANY, ZOLL Medical Corporation (Asahi Kasai Company), AliveCor Inc., Bexen Cardio, iRhythm Technologies, Inc., Metrax GmbH, Koninklijke Philips N.V., Osypka Medical GmbH, Abbott, among others.
Respiratory Monitoring Devices Market
Respiratory Monitoring Devices Market Insights, Competitive Landscape, and Market Forecast – 2032 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key respiratory monitoring devices companies, including Koninklijke Philips NV, ResMed, Medtronic plc., GE Healthcare, Draegerwerk, Seimens Healthineers, Nihon Kohden Corporation, Vyaire Medical, Inc., Hamilton Medical, Getinge Inc., BD, Airsep Corporation, Smiths Medical, Masimo, Fisher and Paykel Healthcare, among others.
Hemodynamic Monitoring Systems Market
Hemodynamic Monitoring Systems Market Insights, Competitive Landscape, and Market Forecast – 2032 report deliver an in-depth understanding of the market trends, market drivers, market barriers, and key hemodynamic monitoring systems companies, including Edwards Lifesciences Corporation, Getinge AB, Deltex Medical Limited, ICU Medical, Inc., CNSystems Medizintechnik GmbH, Caretaker, LLC, Osypka Medical GmbH, Baxter International Inc (Cheetah Medical, Inc.), Shenzhen Comen Medical Instruments Co., Ltd., General Meditech Inc., Drägerwerk AG & Co. KGaA, General Electric Company, Smiths Group, Transonic Systems Inc., EMTEL Śliwa sp. K, Koninklijke Philips N.V., NIMedical, Masimo (LiDCO Group Plc), Nihon Kohden Corporation, Hemo Sapiens Inc., among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Pipeline Assessment
Healthcare Licensing Services
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.
CONTACT: Contact Us Shruti Thakur [email protected] +14699457679
 Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. NewIndiaObserver.com takes no editorial responsibility for the same.
Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. NewIndiaObserver.com takes no editorial responsibility for the same.
