Life Science CDMO Market Outlook 2025–2034: Trends, Growth Drivers and Regional Insights

This report, published by Towards Healthcare, a sister firm of Precedence Research, provides an in-depth analysis of the global life science CDMO market. It highlights key trends, regional dynamics, and growth opportunities shaping the industry’s future from 2025 to 2034.
Ottawa, Oct. 28, 2025 (GLOBE NEWSWIRE) — The global life science CDMO market is witnessing substantial growth, with revenues projected to reach several hundred million dollars by 2034. This expansion is fueled by rising outsourcing demand within the biotechnology and pharmaceutical sectors.
North America currently leads the market, driven by a growing pipeline of personalized medicines, biologics, and complex therapies such as cell and gene treatments. Meanwhile, Asia Pacific is emerging as the fastest-growing region, supported by robust R&D infrastructure and increasing healthcare investments.
The major factors driving market growth are the growing research and development activities and technological innovation. Large pharmaceutical companies outsource their research and manufacturing services to accelerate the development of drugs or medical devices. The increasing collaborations and investments by government and private organizations also contribute to market growth.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/5900
The Life Science CDMO Market: Highlights
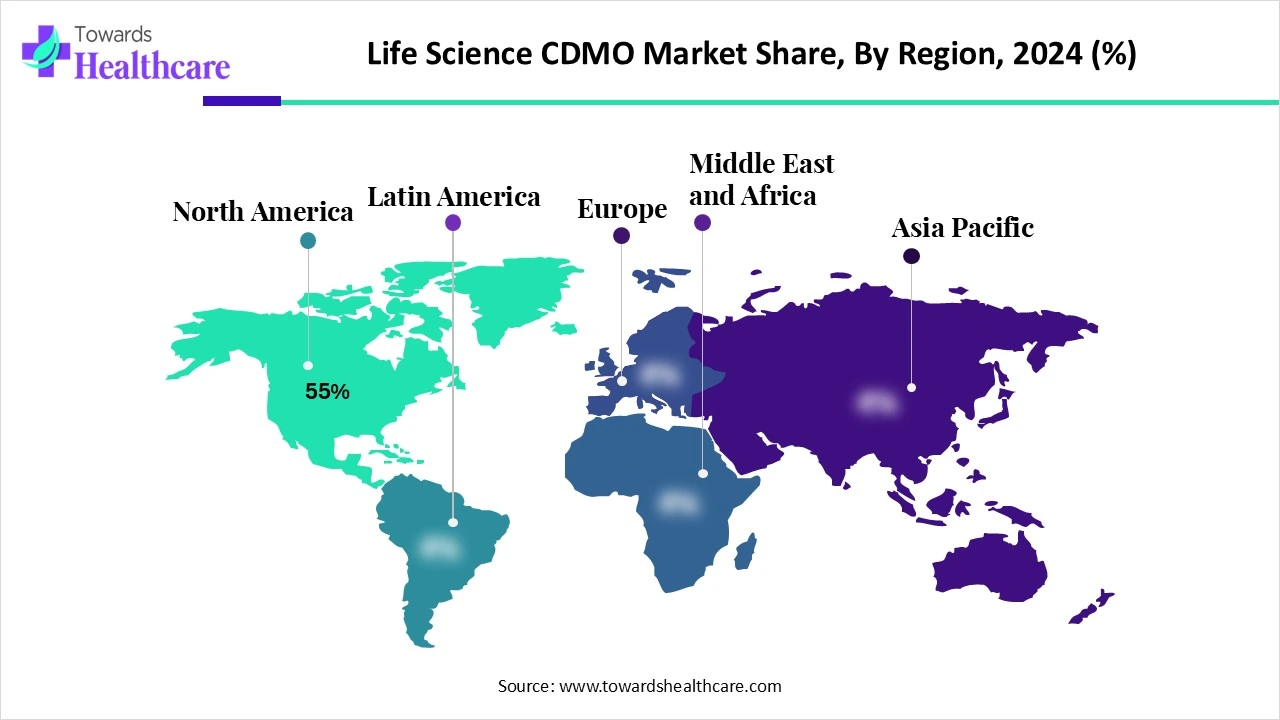
- North America dominated the global market by 55% in 2024.
- Asia-Pacific is expected to grow at the fastest CAGR in the market during the forecast period.
- By service type, the API development and manufacturing segment accounted for the highest revenue share of the market in 2024.
- By service type, the biologics CDMO services segment is expected to witness the fastest growth in the market over the forecast period.
- By phase, the commercial manufacturing segment led the market in 2024.
- By phase, the preclinical development services segment is expected to show the fastest growth in the life science CDMO market in the upcoming years.
- By customer type, the large pharma companies segment held a major revenue share of the market in 2024.
- By customer type, the small & mid-size biotech firms segment is expected to grow with the highest CAGR in the market during the studied years.
- By molecule type, the small molecule segment registered its dominance over the global market in 2024.
- By molecule type, the biologics segment is expected to expand rapidly in the market in the coming years.
What is Life Science CDMO?
The life science CDMO market refers to an organization that provides a wide range of services, from research to regulatory support, to pharmaceutical and biotechnology companies. CDMOs streamline the drug development and manufacturing process, enabling faster, more efficient, and cost-effective production of pharmaceutical products. They eliminate the need for a life science company to purchase expensive, specialized equipment and make large capital investments in developing favorable infrastructure.
You can place an order or ask any questions, please feel free to contact us at [email protected]
Technological Advancements: Major Potential
The future of the life science CDMO market is promising, driven by the integration of artificial intelligence (AI) and machine learning (ML) to revolutionize processes. AI and ML help CDMOs enhance process optimization, reduce costs, improve product quality, and increase production rates. They introduce automation in every task and reduce manual errors. They analyze client-provided data and draw conclusions based on different experiments, providing customized solutions. Additionally, CDMOs adopt green manufacturing practices to comply with environmental sustainability.
Regulatory Challenges: Major Limitation
Life science CDMOs face challenges related to evolving regulatory landscapes, leading to slower product development. Poor data integrity, lack of validation, inadequate quality management, and non-compliance with global regulations can lead to increased costs. CDMOs need to comply with the regulatory policies of different nations.
The Life Science CDMO Market: Regional Analysis
North America held a major revenue share of the market in 2024. North America has a strong presence of pharmaceutical and biotechnology companies that focus on the development of innovative products. The growing demand for personalized medicines, increasing market competition, and favorable regulatory support propel the market in North America. Government organizations provide funding for conducting research and manufacturing activities. The market experiences robust growth due to increasing collaborations among key players and the rising adoption of advanced technologies.
Life science CDMOs in the U.S. are expanding rapidly due to biotech innovation, increased demand for biologics, strong regulatory support, and venture capital investments, fueling domestic manufacturing and service partnerships.
Canada’s CDMO sector is growing steadily, driven by government incentives, a skilled workforce, and increasing demand for cell and gene therapies. Strategic investments are enhancing domestic capabilities in biomanufacturing and R&D services.
Asia-Pacific is expected to host the fastest-growing market in the coming years. The life science CDMO market in Asia-Pacific is driven by the burgeoning life science sector, the increasing number of life science startups, and favorable government support. Asia-Pacific countries have a suitable manufacturing infrastructure, encouraging foreign companies to set up their manufacturing facilities. The availability of cost-effective infrastructure and skilled professionals facilitates market growth.
China’s CDMO market is booming with strong government backing, low manufacturing costs, and rising biotech startups. Global companies are outsourcing to China for efficient, scalable, and cost-effective pharmaceutical development solutions.
India’s life science CDMO industry thrives on affordability, technical expertise, and global partnerships. Growing biologics demand, favorable policies, and strong infrastructure are positioning India as a key global outsourcing hub.
Download the single region market report @ https://www.towardshealthcare.com/checkout/5900
The Life Science CDMO Market: Segmentation Analysis
By Service Type
The API development and manufacturing segment held a dominant presence in the market in 2024, due to the rising prevalence of chronic disorders and growing demand for personalized medicines. API development is a complex process that requires significant time and capital expenditure. It generally takes 12-15 years and costs approximately 2-3 billion. Thus, life science companies collaborate with CDMOs to expedite the entire development and manufacturing process, reducing the time-to-market approval.
The biologics CDMO services segment is expected to grow at the fastest CAGR in the market during the forecast period. Biologics are widely preferred as they provide targeted treatment with reduced systemic side effects. Biologics CDMOs have specialized infrastructure and equipment to develop novel biologics based on patients’ conditions. Their end-to-end solutions enable companies to advance their therapies to patients. The increasing use of single-use technologies for fermentation technologies boosts the segment’s growth.
By Phase
The commercial manufacturing segment held the largest revenue share of the life science CDMO market in 2024, due to the growing need for the timely delivery of pharmaceutical products. CDMOs have scalable manufacturing facilities that lead to rapid timelines and flexible batch sizes. They act as strategic partners to pharmaceutical companies to bring therapies to market efficiently while managing risks and costs. They expedite the manufacturing process, reducing time spent on facility setup and validation.
The preclinical development services segment is expected to grow with the highest CAGR in the market during the studied years. CDMOs support early drug development and preclinical requirements. They help develop pharmaceutical products and enable sourcing, regulatory, and laboratory testing requirements. Pharmaceutical products are developed for animal testing to assess their toxicity profiles and efficacy. CDMOs deliver high-quality results for narrowing the selection of drug candidates in the preclinical drug development process.
Become a valued research partner with us – https://www.towardshealthcare.com/schedule-meeting
By Customer Type
The large pharma companies segment contributed the biggest revenue share of the life science CDMO market in 2024, due to increasing competition among key players and the growing need to serve a large patient population. Large pharmaceutical companies focus on multiple projects simultaneously. This necessitates them to outsource their research requirements to a third-party organization that helps expedite product development. The increasing competition among large companies potentiates the need to expand their product pipelines and reduce the time to market approval.
The small & mid-size biotech firms segment is expected to expand rapidly in the market in the coming years. The increasing number of life science startups and the increasing funding to small- and medium-sized enterprises (SMEs) facilitate them to collaborate with CDMOs. SMEs lack sufficient infrastructure for the development and large-scale manufacturing of pharmaceutical products. SMEs collaborate with CDMOs as they provide relevant expertise and access to specialized facilities.
By Molecule Type
The small molecule segment led the life science CDMO market in 2024, due to higher efficacy and bioavailability. Small molecules are easily absorbed by the body and distributed through the blood circulation, potentiating their biological effect. They are comparatively less complex and do not require expensive manufacturing equipment for their development. They are cost-effective and can be administered through the oral route, enhancing patient medication adherence and convenience.
The biologics segment is expected to witness the fastest growth in the market over the forecast period. Biologics are large, complex molecules derived from patients’ genetic profiles. This leads to personalized treatment, enhancing efficiency and reducing side effects. Biologics can treat a wide range of disorders that are otherwise difficult to treat with small molecules, including genetic and rare diseases. They cure a disease from its root cause, reducing the risk of recurrence.
Become a valued research partner with us – https://www.towardshealthcare.com/schedule-meeting
Top Companies and Their Contributions to the Market
| Company | Offerings & Contributions |
| Lonza Group | Provides end-to-end services in biologics, small molecules, cell & gene therapies. Renowned for commercial-scale manufacturing and regulatory expertise. |
| Thermo Fisher Scientific | Offers comprehensive CDMO services via Patheon, including API development, biologics, sterile injectables, and gene therapy manufacturing. |
| Samsung Biologics | Specializes in large-scale biologics manufacturing, biosimilars, and end-to-end services from cell line development to fill-finish. |
| Fujifilm Diosynth Biotechnologies | Provides biologics and viral vector development/manufacturing. Strong in cell culture, microbial fermentation, and gene therapies. |
| Piramal Pharma Solutions | Delivers integrated drug development and manufacturing for APIs and formulations, with global facilities focused on niche high-potency compounds. |
| AGC Biologics | Offers biologics CDMO services, including mammalian and microbial systems, with expertise in cell therapy and plasmid DNA production. |
| Almac Group | Provides pharmaceutical development, clinical trial supply, and commercial manufacturing with a strong focus on quality and regulatory compliance. |
| Evonik Health Care | Specializes in complex APIs, drug delivery systems, and formulation development, especially for parenteral and oral dosage forms. |
| Syngene International | Offers integrated research and development services across biologics and small molecules with strong capabilities in discovery to commercial scale. |
| Therapure Biopharma | Focuses on biologics manufacturing, including plasma-derived therapeutics, with expertise in process development and aseptic fill-finish. |
Browse More Insights of Towards Healthcare:
The global life science consulting market was valued at USD 34.82 billion in 2024, grew to USD 38.08 billion in 2025, and is anticipated to reach USD 84.83 billion by 2034, expanding at a CAGR of 9.38% during 2025–2034.
North America Life Science Market dominated the landscape, with its market size estimated at USD 35.42 billion in 2024, rising to USD 39.25 billion in 2025, and projected to hit USD 110.75 billion by 2034, advancing at a robust CAGR of 12.08%.
The Europe life science market was valued at USD 26.32 billion in 2024, grew to USD 29.40 billion in 2025, and is forecast to reach USD 78.90 billion by 2034, expanding at a CAGR of 11.61%.
The Asia-Pacific (APAC) life science market reached USD 17.51 billion in 2024 and is expected to grow to USD 19.46 billion in 2025, ultimately surpassing USD 54.81 billion by 2034 at a healthy CAGR of 12.09%.
Meanwhile, the Middle East and Africa (MEA) life science market accounted for USD 4.47 billion in 2024, rising to USD 5.04 billion in 2025, and projected to reach USD 13.07 billion by 2034, growing at a CAGR of 11.32%.
The Latin America life science market stood at USD 4.49 billion in 2024, increased to USD 4.90 billion in 2025, and is expected to exceed USD 12.05 billion by 2034, registering a CAGR of 10.38%.
The U.S. life science market is on an upward trajectory, set to generate substantial revenue growth and climb into the hundreds of millions of dollars over the forecast period from 2025 to 2034.
Additionally, the global life science consumables market was valued at USD 29.88 billion in 2024, increased to USD 31.51 billion in 2025, and is projected to reach USD 50.71 billion by 2034, advancing at a CAGR of 5.44%.
The life sciences ERP software market is also gaining momentum worldwide, with expectations of generating hundreds of millions in revenue between 2025 and 2034.
Moreover, the global life science equipment market was valued at USD 63.72 billion in 2024, grew to USD 67.76 billion in 2025, and is forecast to reach USD 120.82 billion by 2034, expanding at a CAGR of 6.34% over the same period.
Recent Developments in the Life Science CDMO Market
- In June 2025, Zydus Lifesciences Ltd. announced the acquisition of Agenus, Inc.’s U.S.-based CMC facilities to enter the global CDMO business. The acquisition was made with an upfront payment of $75 million and a contingent payment of $50 million for over three years. This expands the presence of Zydus in California and provides access to advanced biologics manufacturing capabilities.
- In September 2024, Serán Bioscience announced that it raised $200 million to build a new commercial-scale manufacturing facility. The new facility will enable the company to provide integrated capabilities across multiple drug delivery and final dose formats. The new facility is estimated to be completed in 2026 and is leveraged with advanced technologies.
- In February 2024, Suven Pharmaceuticals Ltd announced a collaboration with Cohance Lifesciences Ltd to merge their businesses and strengthen their position in the CDMO market. The collaboration will help Suven become a diversified CDMO and API leader in India, transcending its current revenue base.
Life Science CDMO Market Top Companies
- Lonza Group
- Thermo Fisher Scientific
- Samsung Biologics
- Fujifilm Diosynth Biotechnologies
- Piramal Pharma Solutions
- AGC Biologics
- Almac Group
- Evonik Health Care
- Syngene International
- Therapure Biopharma
- WuXi Biologics
Download the Competitive Landscape market report @ https://www.towardshealthcare.com/checkout/5900
The Life Science CDMO Market Segmentation
By Service Type
- API Development & Manufacturing
- Small molecules, high potency APIs (HPAPIs), intermediates
- Biologics CDMO Services
- Monoclonal antibodies, recombinant proteins, vaccines
- Finished Dosage Form (FDF) Manufacturing
- Oral solids, injectables, topicals, ophthalmics
- Cell & Gene Therapy Services
- Viral vectors, plasmids, CAR-T support
- Analytical & Regulatory Services
- Bioanalytical testing, regulatory consulting, QC release
By Phase
- Commercial Manufacturing
- Preclinical Development Services
- Clinical-Stage Manufacturing
By Customer Type
- Large Pharma Companies
- Small & Mid-size Biotech Firms
- Virtual Pharma & Startups
By Molecule Type
- Small Molecules
- Biologics
- Advanced Therapies
By Region
- North America
- U.S.
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
- Kuwait
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/checkout/5900
Access our exclusive, data-rich dashboard dedicated to the healthcare market – built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at [email protected]
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire | Nutraceuticals Func Foods | Onco Quant | Sustainability Quant | Specialty Chemicals Analytics
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest
 Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. NewIndiaObserver.com takes no editorial responsibility for the same.
Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. NewIndiaObserver.com takes no editorial responsibility for the same.
